The Architecture of Change: A Deep Dive into Basic Chemical Reactions
At its core, chemistry is the science of change. It is the study of how substances, known as reactants, transform into entirely new substances, the products, through the intricate dance of breaking and forming chemical bonds. [1][2] This process, a chemical reaction, is the engine of the material world, driving everything from the metabolic pathways that sustain life to the industrial processes that build our society. [2][3] While the universe of possible reactions is virtually infinite, a foundational understanding can be achieved by classifying them into a few principal types. A thorough examination of these archetypes—synthesis, decomposition, single- and double-displacement, and combustion—reveals the elegant logic governing the transformation of matter.
Synthesis and Decomposition: The Yin and Yang of Molecular Construction
Synthesis and decomposition reactions represent two sides of the same coin: the former builds complexity, while the latter breaks it down. [4][5] A synthesis, or combination, reaction occurs when two or more simpler substances merge to form a single, more complex product. [6] This process is fundamental to chemical manufacturing, enabling the creation of everything from pharmaceuticals to advanced materials. [7][8] A classic industrial example is the Haber-Bosch process, which synthesizes ammonia (NH₃) from nitrogen and hydrogen gas, a cornerstone for the production of agricultural fertilizers. [8] Another illustration is the reaction of quicklime (calcium oxide, CaO) with water to form slaked lime (calcium hydroxide, Ca(OH)₂), a compound used in food processing, paper manufacturing, and even dental treatments. [9][10] Many synthesis reactions are exothermic, releasing energy as new, more stable bonds are formed. [4]
Conversely, decomposition reactions involve a single compound breaking down into two or more simpler substances. [11] This process typically requires an input of energy—such as heat (thermal decomposition), electricity (electrolytic decomposition), or light (photolysis)—to break the existing chemical bonds. [5][12] The electrolysis of water into hydrogen and oxygen gas is a prime example of electrolytic decomposition, demonstrating how energy can reverse a synthesis reaction. [13] Thermal decomposition is common in both industry and nature; heating calcium carbonate (limestone) causes it to break down into calcium oxide and carbon dioxide, a key step in cement production. [5][14] In nature, the decomposition of organic matter by microorganisms is a complex series of reactions essential for recycling nutrients like carbon and nitrogen back into the ecosystem. [14] Understanding the activation energy required to initiate decomposition is critical for both leveraging these reactions and preventing undesired breakdown of materials. [5][12]
Displacement Reactions: The Dynamics of Ionic Exchange
Displacement reactions are defined by the replacement of one element or group by another within a compound. They are broadly categorized as single-displacement and double-displacement reactions. In a single-displacement reaction, a more reactive element displaces a less reactive element from a compound. [15] The viability of these reactions can be predicted using an activity series, which ranks elements based on their reactivity. [16][17] For instance, if a piece of zinc metal is placed in a copper(II) sulfate solution, the more reactive zinc will displace the copper, forming zinc sulfate and solid copper. [16] This principle has practical applications, such as using a more reactive metal for sacrificial corrosion protection. Conversely, a less reactive element like gold will not react with acids to displace hydrogen, which contributes to its stability and use in jewelry. [18]
Double-displacement reactions, also known as metathesis, involve the exchange of ions between two aqueous ionic compounds to form two new compounds. [19][20] These reactions are ubiquitous in solution chemistry and often manifest in two prominent forms: precipitation and neutralization. [21][22] A precipitation reaction occurs when the exchanged ions form an insoluble solid, or precipitate. [23] For example, mixing solutions of silver nitrate and sodium chloride results in the formation of insoluble silver chloride, which visibly precipitates out of the solution. [21] This type of reaction is crucial for processes like water treatment, where it can be used to remove unwanted ions. [21] Neutralization is a specific type of double-displacement reaction between an acid and a base, which produces a salt and water. [19][21] The reaction between hydrochloric acid (a strong acid) and sodium hydroxide (a strong base) to form sodium chloride and water is a quintessential neutralization reaction that demonstrates the exchange of H⁺ and OH⁻ ions. [20]
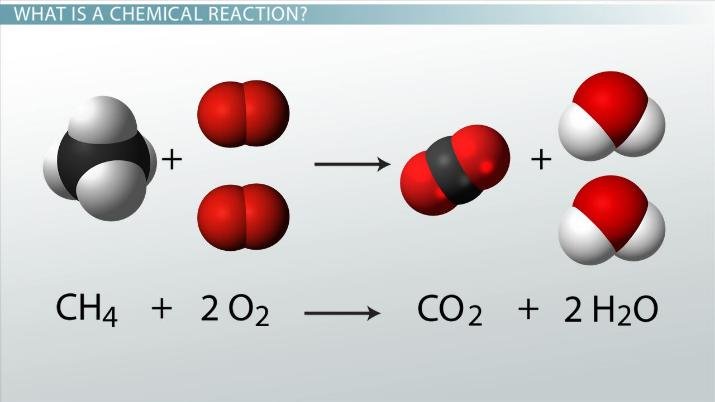
Combustion and the Overarching Framework of Redox
Combustion is a rapid, exothermic reaction between a substance and an oxidant, usually oxygen, that produces heat and light. [6][24] For hydrocarbons—compounds of carbon and hydrogen—complete combustion yields carbon dioxide and water. [6] This process is the primary mechanism for energy generation from fossil fuels and biofuels, powering vehicles and power plants. [25][26] The efficiency and products of combustion are governed by principles of thermodynamics, which determine the potential energy release, and kinetics, which dictate the reaction rate. [24][27] Incomplete combustion, which occurs with insufficient oxygen, can produce harmful byproducts like carbon monoxide. [28] While often viewed as its own category, combustion is also a prime example of an oxidation-reduction (redox) reaction. [26][29]
Redox reactions are fundamental to nearly all major reaction types and are defined by the transfer of electrons between chemical species. [3] One substance loses electrons (oxidation), while another gains them (reduction). [26] This electron transfer is evident in many everyday processes. The rusting of iron is a redox reaction where iron is oxidized by oxygen. [25] Biological processes like photosynthesis and cellular respiration are complex series of redox reactions; in respiration, glucose is oxidized to release energy for bodily functions. [3][30] Even batteries function based on controlled redox reactions to generate an electric current. [3] Recognizing the redox nature of reactions like synthesis, decomposition, single-displacement, and combustion provides a deeper, more unified understanding of chemical change, revealing the electron as a primary currency of chemical transformation.