The Atom and Its Components: An Inquiry into the Essence of Matter
The atom, a term derived from the Greek ‘atomos’ meaning indivisible, represents the fundamental unit of a chemical element. [1][2] This seemingly simple definition belies a universe of complexity, governed by intricate laws and forces that dictate the very nature of existence. An exploration of the atom and its constituents—protons, neutrons, and electrons—is not merely a study of particles but a journey into the core principles of physics and chemistry, revealing how matter is constructed, how it interacts, and how it releases immense energy. This report delves into the sophisticated architecture of the atom, the dynamic interplay of the forces that bind it, and the profound implications of its composition, offering a detailed and factual examination of these building blocks of reality.
The Nuclear Core and the Electron Cloud: A Duality of Mass and Influence
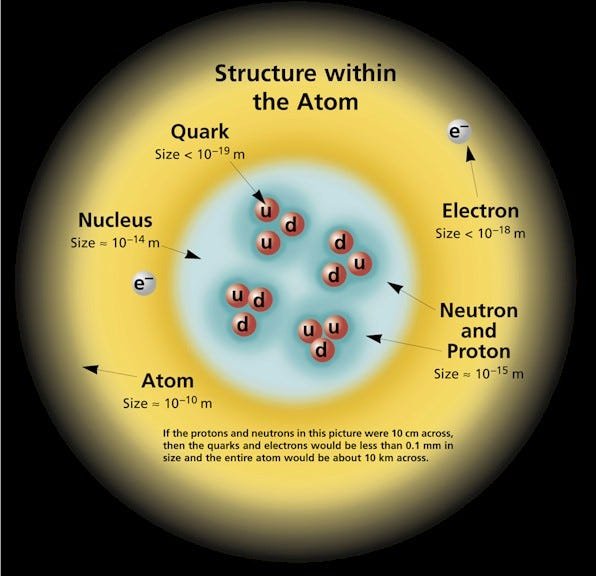
At the heart of every atom lies the nucleus, a region of incredible density containing positively charged protons and neutral neutrons. [3][4] This central body, though occupying a minuscule fraction of the atom’s total volume, accounts for over 99.9% of its mass. [5] The number of protons, known as the atomic number (Z), is the defining characteristic of an element; an atom with six protons will always be carbon, while one with 79 will always be gold. [3][4] Neutrons, having no charge, do not alter the chemical identity but do affect the mass, creating different forms of an element known as isotopes. [4][6] For example, Carbon-12, with six protons and six neutrons, is a stable isotope, whereas Carbon-14, with six protons and eight neutrons, is radioactive and famously used in archaeological dating. [7][8] Surrounding this dense core is a vast, nebulous region occupied by negatively charged electrons. [3][6] These electrons, despite their negligible mass, are paramount in defining an atom’s chemical behavior. [9][10] It is the arrangement of these valence electrons—those in the outermost shell—that dictates how an atom will bond with others, either by transferring or sharing electrons to form the vast array of molecules and compounds that constitute the world. [11][12]
The Fundamental Forces: An Orchestra of Interaction
The stability and structure of the atom are maintained by a delicate and powerful interplay of fundamental forces. The electromagnetic force governs the attraction between the negatively charged electrons and the positively charged nucleus, holding the atom together. [13] However, this same force creates a powerful repulsion between the like-charged protons crammed into the nucleus. [13][14] Overcoming this immense repulsion is the strong nuclear force, a fundamental interaction approximately 100 times stronger than electromagnetism at subatomic distances. [13][14] This force, mediated by particles called gluons, binds protons and neutrons (collectively known as nucleons) together, creating a stable nucleus. [14][15] The sheer energy associated with the strong force is the reason nuclear fuel has an energy density millions of times greater than chemical fuels. [13] A third force, the weak nuclear force, operates at an even shorter range and is responsible for radioactive decay. [16][17] It is unique in its ability to change the “flavor” of quarks, the fundamental particles that make up protons and neutrons. [16][18] This process, known as beta decay, can transform a neutron into a proton (or vice versa), emitting an electron and an antineutrino in the process. [16][19] This transformative power is not only crucial for allowing unstable isotopes to decay into more stable configurations but also drives the nuclear fusion processes that power the sun and stars. [17][20]
Mass Defect and Binding Energy: The Tangible Effects of Force
The power of the strong nuclear force has a directly observable and counterintuitive consequence: the mass of a stable nucleus is always less than the sum of the individual masses of its constituent protons and neutrons. [21][22] This “missing mass” is known as the mass defect. [23][24] It is not truly lost but is converted into a tremendous amount of energy, known as the nuclear binding energy, in accordance with Albert Einstein’s famous equation, E=mc². [21][22] This binding energy represents the energy released when the nucleus was formed and, conversely, is the minimum energy required to break the nucleus apart into its individual nucleons. [23][24] A higher binding energy per nucleon corresponds to a more stable nucleus. [25] This principle is the bedrock of nuclear energy. In nuclear fission, a heavy, less stable nucleus like Uranium-235 splits into lighter, more stable nuclei, releasing a portion of this binding energy. [22] In nuclear fusion, light nuclei, like isotopes of hydrogen, combine to form a heavier, more stable nucleus (like helium), also resulting in a massive release of energy because the resulting nucleus has a higher binding energy per nucleon. [15][22] This process, which powers our sun, demonstrates how the fundamental forces within the atom translate directly into the vast energy that shapes the cosmos. [15][17]
The Standard Model: A Deeper Layer of Reality
The discovery of protons, neutrons, and electrons was a monumental leap, but the journey into the atom’s structure did not end there. The Standard Model of Particle Physics, developed throughout the 20th century, reveals that even protons and neutrons are not fundamental. [20][26] They are composite particles called hadrons, each made up of three smaller, elementary particles known as quarks. [15][27] Quarks come in six “flavors,” but all ordinary matter is composed of just two: up quarks and down quarks. [20][27] A proton consists of two up quarks and one down quark, while a neutron has one up quark and two down quarks. [28] This composition explains their respective electrical charges. Electrons, in contrast, are truly fundamental particles belonging to a class called leptons. [27][29] The Standard Model categorizes all known elementary particles and describes three of the four fundamental forces through which they interact, mediated by force-carrying particles called bosons (gluons for the strong force, W and Z bosons for the weak force, and photons for the electromagnetic force). [20][27] This framework represents our most profound understanding of the universe’s basic constituents, a testament to a scientific journey that began with the philosophical idea of an “uncuttable” particle and has culminated in a detailed map of the subatomic realm. [1][30]